Asymmetric synthesis of spirooxindole–pyranoindole products via Friedel–Crafts alkylation/cyclization of the indole carbocyclic ring†
Abstract
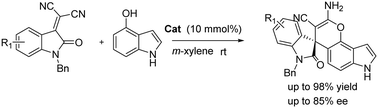
An efficient enantioselective Friedel–Crafts alkylation/cyclization tandem reaction of the indole carbocyclic ring with isatylidene malononitriles has been performed successfully by using a new bifunctional tertiary amine-urea catalyst. A series of chiral spirooxindole–pyranoindole products were obtained in excellent yields (up to 98% yield) with moderate to good enantioselectivities (up to 85% ee).


 Please wait while we load your content...
Please wait while we load your content...