Water soluble palladium(ii) and platinum(ii) acyclic diaminocarbene complexes: solution behavior, DNA binding, and antiproliferative activity†
Abstract
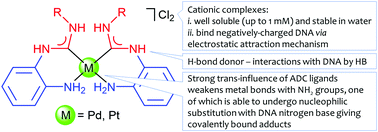
Bis-ADC complexes cis-[Pd{C(NHC6H4NH2)N(H)R}2]Cl2 (R = Xyl 4a, Cy 4b, C6H4-4-F 4c) and cis-[Pt{C(NHC6H4NH2)N(H)R}2]Cl2 (R = Xyl 5a, Cy 5b, C6H4-4-F 5c) were synthesized via the metal-mediated coupling of two isocyanide ligands in cis-[MCl2(CNR)2] (M = Pd, Pt; R = Xyl, Cy, C6H4-4-F) and 1,2-diaminobenzene. New compounds 4c and 5a–c were characterized by HR ESI+-MS, IR, and 1H, 13C{1H} and 195Pt{1H} NMR spectroscopy; the structures of 4a and 5a were elucidated by single-crystal X-ray diffraction. The stability of the ADC complexes in aqueous media (5 mM NaCl) was monitored by UV absorption spectroscopy, HR ESI+ mass spectrometry, and 195Pt{1H} NMR spectroscopy (for 5a). Molar conductivity measurements in MeOH (ΛM = 167–173 Ω−1 mol−1 cm2) indicate that, in this solvent, the ADC complexes exist as dicationic species of [A][Q]2 type. The ADC complexes binding to CT DNA was investigated by means of spectroscopic and hydrodynamic techniques including UV absorption and circular dichroism spectroscopy, fluorescence spectroscopy, low-gradient viscometry, flow birefringence, and AFM imaging. As a result, complexes 4a and 5a were shown to bind double-stranded DNA predominantly via the formation of monofunctional adducts in the major groove of the macromolecule. Binding of the ADC complexes also provokes the formation of a large number of intermolecular DNA–DNA contacts in solution. The antiproliferative activity of all prepared ADC complexes 4a–c and 5a–c was evaluated in vitro against three human carcinoma cell lines (HT-29, MDA-MB-231, and MCF-7) and two non-tumorigenic cell lines (L929 and RC-124) and compared to that of cisplatin. Among the compounds studied, complexes 4a and 5a appeared to be the most active species with IC50 values in MCF-7 cells of about 10 μM.


 Please wait while we load your content...
Please wait while we load your content...