Electrode processes in the KF–AlF3–Al2O3 melt
Abstract
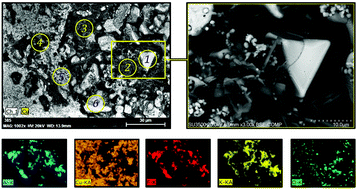
The kinetics of anodic and cathodic processes in KF–AlF3 and KF–AlF3–Al2O3 melts obtained by means of stationary polarization and cyclic voltammetry have been studied. Studies were conducted at different cryolite ratios [CRs, (moles of KF)/(moles of AlF3)] and different alumina contents at 700–800 °C for the Cu–Al anode and at 800 °C for the tungsten cathode. The limiting current density of oxygen evolution on the Cu–Al anode was 0.31 A cm−2 at 700 °C and CR = 1.4. It drastically increases with CR and temperature. The limiting current density of aluminium reduction on the W cathode was in the range 0.45–0.50 A cm−2. The cathodic process is diffusion-controlled while the anodic one shows mixed kinetics. The diffusion and mass transfer coefficients for the cathodic process (W electrode) and the mass transfer coefficient for the anodic process (Cu–Al electrode) were determined. Based on the obtained results, the electrolysis of 1.4KF–AlF3–Al2O3(sat) with the Cu–Al anode and W cathode was performed. The aluminium purity was determined using an optical emission spectrometer. The XRD and SEM-EDX methods were used to study the phases formed on the anode surface.


 Please wait while we load your content...
Please wait while we load your content...