Synthesis of an amino phosphinodiselenoic acid ester and β-amino diselenides employing P2Se5†
Abstract
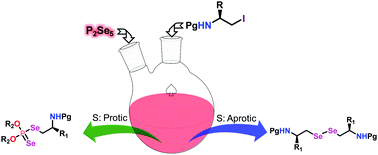
The synthesis of a new class of amino phosphinodiselenoic acid ester and β-amino diselenides is conducted by employing a reaction between Nβ-protected aminoalkyl iodide and phosphorus pentaselenide (P2Se5) has been described. In the presence of protic solvents, amino phosphinodiselenoic acid esters were found to be the major products, whereas β-amino diselenides were formed exclusively when the reaction was carried out in polar aprotic solvents.


 Please wait while we load your content...
Please wait while we load your content...