Fabrication of a novel Ni3N/Ni4N heterojunction as a non-noble metal co-catalyst to boost the H2 evolution efficiency of Zn0.5Cd0.5S†
Abstract
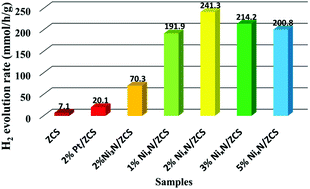
Previous studies have shown that auxiliary catalysts play a significant role in improving the activity and reliability of semiconductor photocatalysis for photocatalytic H2 production. However, designing an efficient and low-cost auxiliary catalyst to replace expensive noble metals has been a major challenge. In our study, we synthesized a non-noble metal co-catalyst Ni3N/Ni4N (NixN) by a two-step method. Upon loading 2% NixN on Zn0.5Cd0.5S (ZCS), the photocatalytic activity of 2% NixN/ZCS greatly improved. The corresponding H2 evolution rate was about 241.3 mmol h−1 g−1, which was obtained in 0.35 M Na2S/0.25 M Na2SO3. This value is about 33 times higher than that of pure ZCS, 12 times higher than that of 2% Pt on ZCS and 3 times higher than that of 2% Ni3N/ZCS. When the concentration of Na2S/Na2SO3 was increased to 1.05 M/0.75 M, the H2 production rate of 2% NixN/ZCS reached 297.6 mmol h−1 g−1 under visible light irradiation (λ > 420 nm), and the quantum efficiency (QE) was 43.8% at 420 nm. The H2 production efficiency of 2% NixN/ZCS is the highest H2 evolution efficiency among ZCS-based photocatalysts reported so far. The introduction of the Ni3N/Ni4N heterojunction could efficiently accelerate the migration of photocarriers and delay the recombination of electron–hole pairs.


 Please wait while we load your content...
Please wait while we load your content...