Expedient green-chemistry approaches for a one-pot synthesis of two series of novel 1,5-benzodiazepines via domino reactions†
Abstract
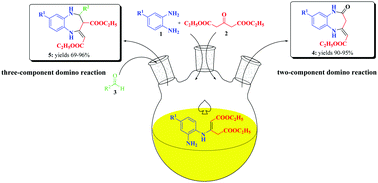
Unique green-chemistry approaches have been developed for the one-pot synthesis of two series of novel 1,5-benzodiazepines 4 and 5. The CeCl3-KI promoted two-component domino reaction of 1,2-phenylenediamines with 1,3-acetonedicarboxylate in ethanol afforded novel 1,5-benzodiazepin-2-ones 4. An expedient approach to 1,5-benzodiazepines 5 has also been developed via three-component domino reactions using 1,2-phenylenediamines, 1,3-acetonedicarboxylate and aldehydes in the presence of a catalytic amount of magnetic nanoparticles (γ-Fe2O3@SiO2/CeCl3) in ethanol at ambient temperature. During the one-pot synthesis, one new seven-membered nitrogen heterocycle (diazepin) and four new bonds (one C–C, two C–N and one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) were constructed by nucleophilic addition, elimination (dehydration, etc.), hydride transfer and cyclization reactions. The structures of all 48 products were fully confirmed by spectroscopic techniques and by single-crystal X-ray analysis for 5db. Moreover, plausible synthesis reaction mechanisms of two series of novel 1,5-benzodiazepines 4 and 5 have been proposed. The methods have advantages such as operational simplicity, mild reaction conditions, short reaction time, easy recovery and reusability of the catalyst, high yields of products, and the use of non-toxic EtOH as the solvent.
C) were constructed by nucleophilic addition, elimination (dehydration, etc.), hydride transfer and cyclization reactions. The structures of all 48 products were fully confirmed by spectroscopic techniques and by single-crystal X-ray analysis for 5db. Moreover, plausible synthesis reaction mechanisms of two series of novel 1,5-benzodiazepines 4 and 5 have been proposed. The methods have advantages such as operational simplicity, mild reaction conditions, short reaction time, easy recovery and reusability of the catalyst, high yields of products, and the use of non-toxic EtOH as the solvent.


 Please wait while we load your content...
Please wait while we load your content...