Unravelling lithiation mechanisms of iron trifluoride by operando X-ray absorption spectroscopy and MCR-ALS chemometric tools†
Abstract
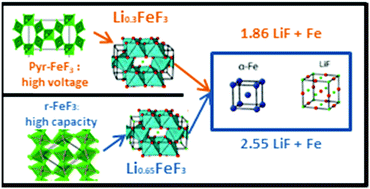
Lithium-ion batteries are extensively used for electrochemical energy storage but their performances need to be continuously increased. One solution is to use alternative cathode materials such as iron trifluoride. However, its galvanostatic profile depends on the allotropic phase studied. To look further into such various lithiation mechanisms, quick-operando XAS has been used upon reduction of anhydrous pyrochlore and rhombohedral iron fluorides. First, fine structural characterization has been conducted by X-Ray Diffraction (XRD), 57Fe Mössbauer and Pair Distribution Function (PDF) analysis. Then, quick operando spectra have been interpreted by MCR-ALS chemometric tools, enabling the identification and quantification of the different phases formed upon insertion and conversion of Fe3+ species.
- This article is part of the themed collection: Sustainability from intercalation compounds


 Please wait while we load your content...
Please wait while we load your content...