Developing a new colorimetric bioassay for iodide determination based on gold supported iridium peroxidase catalysts†
Abstract
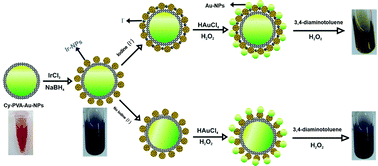
Citrate and polyvinyl alcohol capped gold nanoparticle (Au-NPs-Cy-PVA) and iridium NPs were incorporated by a chemical reduction method and characterized by UV-Vis, DLS, SEM, EDS, XRD, and FT-IR analysis. A cost-effective and straightforward colorimetric sensor dependent on the redox reaction of 3,4-diaminotoluene biomarker (DA) with Au3+ ions and H2O2 on an Au/Ir-NP catalyst mimicking enzyme-like activity, has been manufactured for the determination of I− in media of interest. At the same time, prepared Au/Ir-NPs can catalyze hydrogen peroxide (H2O2), which is used to reduce Au3+ into gold nanoparticles and oxidise 3,4-diaminotoluene biomarker. I− effectively expands the catalytic activity of Au/Ir-nanoparticles to decompose H2O2 by the interaction between Au+ and Au0 and suppresses the oxidation of 3,4-diaminotoluene biomarker. In a framework which contains Au/Ir-NPs, H2O2, 3,4-diaminotoluene biomarker, and HAuCl4, increasing the I− concentration results in color changes from deep purple to yellow. In light of this principle, in this work, a novel colorimetric technique for the sensitive detection of I− ions was developed. This method has a detection limit of 0.058 μM and a linear range between 0.12 to 5.42 μM. The assays are profoundly selective over other ions and can be effectively applied for the determination of iodine ions in real water samples.


 Please wait while we load your content...
Please wait while we load your content...