A novel ratiometric and reversible fluorescent probe based on naphthalimide for the detection of Al3+ and pH with excellent selectivity†
Abstract
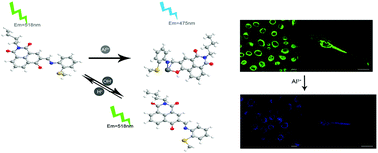
A novel ratiometric and reversible fluorescent probe, (E)-2-butyl-6-hydroxy-5-(((2-(methylthio)phenyl)imino)methyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (denoted as probe L), based on naphthalimide and displaying excellent selectivity and hydrophilicity was designed and synthesized in order to be able to quickly identify Al3+. As the concentration of Al3+ was increased, the probe L emission peak gradually shifted to a lower wavelength (i.e., blue shifted) under single-wavelength excitation. And under UV light, a change in the fluorescence from green to blue was detected by the naked eye. The sensing mechanism was indicated to involve intramolecular charge transfer (ICT). The binding mode of L-Al3+ was investigated by performing Job-plot and density functional theory (DFT) calculations. Probe L also showed a pH-response behavior: when the pH was increased from 8 to 10, the fluorescence intensity of the probe gradually increased, with a particularly good linear relationship. In addition, bioimaging investigations indicated that probe L is membrane permeable and that it can be used to identify Al3+ in zebrafish and HeLa cells.


 Please wait while we load your content...
Please wait while we load your content...