Preparation and characterization of phloretin by complexation with cyclodextrins†
Abstract
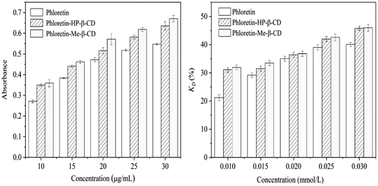
In recent years, phloretin has gained wide interest because of its antioxidant, antitumor, and skin whitening properties. However, its low water solubility and low stability make its usage in clinical application difficult, especially in water-based formulations. Phloretin–methyl-β-cyclodextrin (phloretin–Me-β-CD) and phloretin–(2-hydroxy)propyl-β-cyclodextrin (phloretin–HP-β-CD) inclusion complexes were prepared by the freeze-drying method. Phase solubility studies indicated that the ratio of the host–guest molecules was 1 : 1. The inclusion behavior has been investigated by X-ray diffraction (XRD), scanning electron microscopy (SEM), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), proton nuclear magnetic resonance (1H NMR), and two-dimensional rotational frame nuclear Overhauser effect spectroscopy (2D ROESY). The results showed that the water solubility of phloretin improved after encapsulation. The reducing power (RP) and 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging activity tests revealed that the antioxidant activity of phloretin improved after the formation of inclusion complexes with Me-β-CD and HP-β-CD. This could be a promising way to improve phloretin as a water-based formulation of antioxidants.


 Please wait while we load your content...
Please wait while we load your content...