Synthesis of fused chalcogenophenocarbazoles: towards dual emission resulting from hybridized local and charge-transfer states†
Abstract
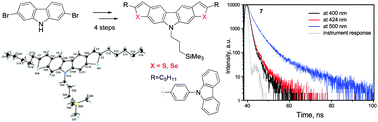
Fused chalcogenophenocarbazoles bearing a trimethylsilylpropyl substituent at the nitrogen atom of carbazole were synthesized starting with 2,7-dibromocarbazole by subsequent alkylation, iodination at positions 3 and 6, Sonogashira coupling and, finally, creation of thiophene and selenophene rings. Depending on the heteroatom and its charge, the compounds were characterized by fluorescence resulting from recombination of either locally excited, intramolecular charge-transfer or hybridized local and charge-transfer (HLCT) states. This is the first report on dual emission of HLCT character observed for chalcogenophenocarbazoles.


 Please wait while we load your content...
Please wait while we load your content...