Hydrothermal synthesis of WS2 quantum dots and their application as a fluorescence sensor for the selective detection of 2,4,6-trinitrophenol†
Abstract
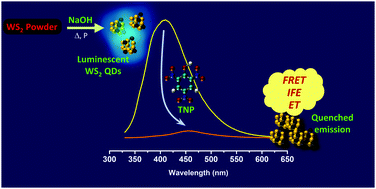
Herein, we report a simple and green approach for the synthesis of photoluminescent WS2 quantum dots (WS2 QDs) from WS2 powder by employing a hydrothermal route in the presence of NaOH. The monodispersed QDs possessed excellent water solubility, good photostability and temporal stability. The selective and sensitive detection of 2,4,6-trinitrophenol (TNP), a member of the family of nitroaromatic (NA) explosives, was demonstrated using the WS2 QDs. Upon interaction with TNP, the PL emission of WS2 QDs was quenched linearly over a range of 0.5 to 95 μM. The limit of detection was as low as 0.30 μM, which attests to the potential of WS2 QDs as a TNP sensor. Electron transfer, Förster resonance energy transfer (FRET) and the inner filter effect (IFE) constituted the mechanism of quenching. Since all NAs are electron deficient, they can facilitate the PL quenching of a fluorophore by electron transfer. However, the latter two quenching mechanisms ensure the selectivity of the QDs towards TNP over other NAs. We envisage these water-soluble QDs as potential chemical sensors in the biological domain.


 Please wait while we load your content...
Please wait while we load your content...