Synthesis of a high-performance low-platinum PtAg/C alloyed oxygen reduction catalyst through the gradual reduction method
Abstract
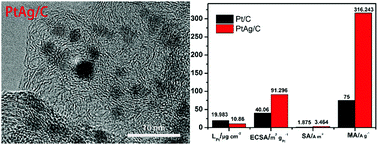
Herein, to reduce the platinum usage and increase the efficiency of noble metals in the catalysis of the oxygen reduction reaction, Ag was used as an assistant reductant, support and sacrificial template to prepare a low-platinum PtAg/C catalyst through a gradual reduction method with the final Pt mass percentage of 10.86 wt%. This as-synthesized low-platinum PtAg/C catalyst exhibited excellent oxygen reduction reaction activity and durability in an acid. With only 54.77% Pt usage, the half-wave potential of PtAg/C is 28 mV higher than that of commercial Pt/C and its limited diffusion current density is 1.1 times that of Pt/C. Furthermore, its electrochemically active surface area is 2.28 times higher than that of commercial Pt/C. Also, its area-specific activity and mass activity are approximately 1.85 and 4.22 times that of Pt/C, respectively. The PtAg/C catalyst displayed extraordinary ORR performance with higher limited current density and half-wave potential than commercial Pt/C after 10 000 potential cycles in HClO4. Thus, PtAg/C is more active and stable for ORR catalysis in acid.


 Please wait while we load your content...
Please wait while we load your content...