New main-group ferrocenyldithiocarbamates and conversion to ferrocene oxazolidine-2-thione and -2-one†
Abstract
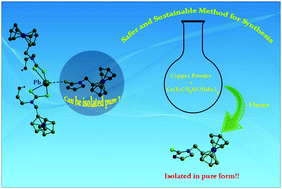
Three new main group ferrocenyldithiocarbamate complexes, viz., {Pb[(FcCH2)(CH2CH2OH)(NCS2)]2}·(FcCH2-3-oxazolidine-2-thione)·(H2O) (1), {Sb[(FcCH2)(CH2CH2OH)(NCS2)]3} (2) and {Bi[(FcCH2)(CH2CH2OH)(NCS2)]3} (3), (Fc = ferrocenyl) have been synthesized and characterized by elemental analyses and IR, 1H and 13C NMR spectroscopy as well as X-ray crystallography. The X-ray analysis of 1 indicates distorted trigonal bipyramidal geometry around Pb(II) and a cyclised product, 3-ferrocenylmethyl-oxazolidine-2-thione (4), co-crystallizes with 1. The geometry around Sb(III) in 2 is pseudo-octahedral, while 3 forms a dimer, displaying distorted square antiprism geometry around the Bi(III) center. Several attempts to synthesize the pure cyclised product 3-ferrocenylmethyl-oxazolidine-2-thione (4) using Pb(II) salts failed but 4 was fortuitously obtained in an attempt to synthesize the Cu(I) cluster by the comproportionation of {Cu[(FcCH2)(CH2CH2OH)(NCS2)]2} using copper powder. Interestingly, varying the reaction time yielded another completely desulfurized cyclised product 3-ferrocenylmethyl-oxazolidine-2-one (5). Both 4 and 5 have been characterized using spectroscopic techniques and X-ray crystallography, and the possible mechanism for the conversion of {Cu[(FcCH2)(CH2CH2OH)(NCS2)]2} to 3-ferrocenylmethyl-oxazolidine-2-thione (4) has been explained with the help of periodic UV-Vis and ESI-MS experiments. This investigation will pave a new pathway for the synthesis of similar cyclised products using similar safe and sustainable reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...