Palladium fabricated on boehmite as an organic–inorganic hybrid nanocatalyst for C–C cross coupling and homoselective cycloaddition reactions†
Abstract
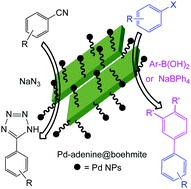
Herein, boehmite nanoparticles were prepared using an aqueous solution of NaOH and Al(NO3)3 9H2O at room temperature. After modification of the boehmite nanoparticle (BNP) surface by 3-choloropropyltrimtoxysilane (CPTMS), adenine was anchored on the surface. Finally, a complex of palladium was fabricated on the BNP surface (Pd-adenine@boehmite). The obtained nanoparticles were identified by TGA, FT-IR, BET, EDS, WDX, SEM, XRD and AAS techniques. In continuation, the Pd-adenine@boehmite was employed as an efficient, reusable and organic–inorganic hybrid catalyst in the C–C cross coupling reactions without a phosphine ligand or an inert atmosphere. Moreover, the homoselective synthesis of tetrazoles was studied in the presence of Pd-adenine@boehmite as a heterogeneous and practical nanocatalyst which can be recovered and reused in the described organic reactions. Besides, organic products which were isolated in suitable TOF and TON numbers in the presence of Pd-adenine@boehmite as a catalyst revealed the practicality of this catalyst. The heterogeneous nature of this catalyst was confirmed by TEM, EDS, WDX, AAS, and FT-IR techniques and, then, compared to the fresh catalyst.


 Please wait while we load your content...
Please wait while we load your content...