Simultaneous formation of non-oxidovanadium(iv) and oxidovanadium(v) complexes incorporating phenol-based hydrazone ligands in aerobic conditions†
Abstract
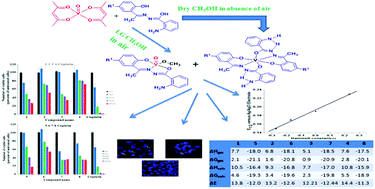
A new family of non-oxidovanadium(IV) [VIV(L)2] (1–4) complexes along with methoxy bonded oxidovanadium(V) [VVO(L)(OCH3)] (5 and 6) and [VVO(L)(OCH3)]2 (7 and 8) complexes have been synthesized by refluxing [VIVO(aa)2] [aa− being the deprotonated form of acetylacetone (Haa)] with a family of hydrazone ligands (H2L1–4, general abbreviation H2L, obtained by the condensation of 2-aminobenzoylhydrazide with 2-hydroxyacetophenone and its 5-substituted derivatives) using laboratory grade methanol as a solvent in the presence of air in ∼30% yield. The DFT calculated changes in Gibbs free energy (ΔG), enthalpy (ΔH) and internal energy (ΔE) for the reaction to form complexes 1–4 in methanol: 2H2L + [VIVO(aa)2] + ¼O2 + CH3OH → [VIV(L)2] + 2Haa + CH3OH + ¼O2 are on average ∼10, ∼5 and ∼14 kcal mol−1 respectively, while these values for the reaction in methanol to form complexes 5–8: 2H2L + [VIVO(aa)2] + ¼O2 + CH3OH → [VVO(L)(OCH3)] + H2L + 2Haa + ½H2O are on average ∼−16, ∼−20 and ∼−12 kcal mol−1 respectively, suggesting that the formation of 1–4 is not thermodynamically feasible through this method. In practice, however, these complexes are also formed probably due to their very low solubility in methanol along with complexes 5–8. The complexes were characterized by analytical and spectral methods. The structures of the H2L2 and H2L3 ligands and complexes 3 and 5–8 were determined by X-ray diffraction. Complexes 1–4 display quasi-reversible one-electron oxidation and reduction peaks in the potential windows of 0.66–0.70 and −0.22 to −0.39 V respectively while complexes 5–8 exhibit quasi-reversible one-electron reduction peaks in the 0.15–0.22 V region. The EPR spectral parameters indicate that the odd electron in complexes 1–4 is present in the dxy orbital, a suggestion which is also supported by a DFT study. The substituents in the aryloxy ring of the hydrazone ligands exhibited a significant effect on λmax for the LMCT transition, A‖, E1/2, ESOMO/EHOMO and ELUMO of the complexes. Complexes 1, 3, and 5–7 showed a wide range of toxicity in a dose dependent manner against lung cancer cells and they kill the cells through apoptosis. The binding ability of the complexes with CT DNA has been determined by a fluorescence emission study and the binding constant value obtained by this method was supported by a molecular docking study.


 Please wait while we load your content...
Please wait while we load your content...