Exploring the mechanism of the Fe(iii)-activated Fenton-like reaction based on a quantitative study†
Abstract
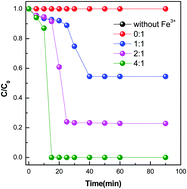
The Fenton method is an important water treatment process. However, the mechanism of the Fenton reaction is still not widely recognized. In this article, the autocatalysis process responsible for the degradation of 4-chlorophenol (4-CP) as well as the generation of Fe(II) and the decomposition of H2O2 is elaborated using a Fe(III)/H2O2 system. It was found that the one electron reduction of Fe(III) to Fe(II) via oxidization of H2O2 to ˙HO2 is not spontaneous according to the standard electrode potential rather than being limited by the rate constant of 0.0027 M−1 s−1 in view of the Haber–Weiss circulation. However, the reaction of Fe(III) and 4-CP is spontaneous with a second-order kinetic rate constant of >0.12 M−1 s−1 and a conversion rate of 0.56%, which triggers the switch of the Fenton-like reaction as a real rate-determining step. The circulation of Fe(III) to Fe(II) in the proposed Fenton-like reaction mechanism depends on the organic species and various intermediates, which indicates its reaction selectivity, preferable for organics with a stronger thermodynamic and kinetic activity. Besides, a slight pH decline with time-scale and the stoichiometric ratio of ˙OH to H2O2 and H2O2 to 4-CP being 1 and 2, respectively, pointed to the new proposed reaction mechanism. The discovery of the new reaction mechanism might promote the theoretical and technical advancement of the Fenton-like process.


 Please wait while we load your content...
Please wait while we load your content...