CHF2CF2OCHF2: conformational analysis and direct dynamics study of its reaction with Cl atoms and atmospheric fate†
Abstract
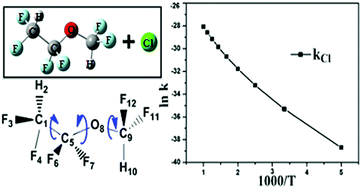
Ten distinct conformers of a CHF2CF2OCHF2 molecule are identified from the exploration of the conformational space and the potential energy profile of the same is constructed from quantum chemical calculations at the M06-2X/6-311++G(d,p) level. Kinetics, mechanism and thermochemistry of the reaction of the two lowest energy conformers of the CHF2CF2OCHF2 molecule with Cl atoms are investigated at the same level of theory. The rate coefficient for the H-abstraction reaction is calculated for the first time using the canonical variational transition state theory (CVT) along with small curvature tunneling (SCT) correction over the temperature range of 200–1000 K and fitted into a three-parameter Arrhenius equation. The subsequent atmospheric degradation of the product radical C˙F2CF2OCHF2 is explored to understand its possible fate and impact in the atmosphere.


 Please wait while we load your content...
Please wait while we load your content...