Theoretical study of the strain effect on the oxygen reduction reaction activity and stability of FeNC catalyst†
Abstract
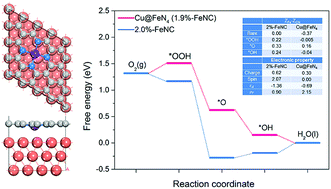
The strain effect on oxygen reduction reaction (ORR) activity and stability of FeNC electrocatalysts has been studied using density functional theory in this work. Based on the reaction free energy results, the rate-determining step (RDS) of ORR on FeNC is *OH → H2O, and the reaction proceeds with the pathway O2 → *OOH → *O → *OH → H2O on the Fe site. Tensile stress is favorable to enhance the ORR activity, and the highest ORR activity is obtained on the 6%-FeNC electrocatalyst. On the other hand, when the whole oxygen reduction process needs synergy between Fe and adjacent C atoms and proceeds with the pathway O2 → *OOH → *TS → *O & #OH → *O → *OH → H2O, the applied stress will decrease the ORR activity of the FeNC electrocatalyst. We further constructed a Cu@FeNC core–shell model to simulate strained FeNC. However, the adsorption energy of ORR intermediates on Cu@FeNC (1.9%-FeNC) is much weaker than on the corresponding 2%-FeNC, mainly due to the coordination between the Cu substrate and Fe atom. In addition, the formation energy and binding energy indicate that neither tensile nor compressive stress is conducive to the stability of the FeNC electrocatalyst. In summary, tensile stress could enhance the ORR activity of FeNC electrocatalysts when ORR occurs on the Fe atom only. What's more, we should pay more attention to the influence of interface interaction if utilizing a M@FeNC model to achieve strained FeNC.


 Please wait while we load your content...
Please wait while we load your content...