Formation, stability and catalase-like activity of mononuclear manganese(ii) and oxomanganese(iv) complexes in protic and aprotic solvents
Abstract
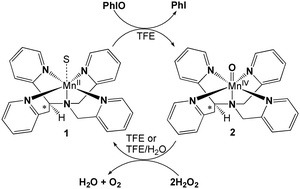
Synthetic compounds as biomimics of catalase enzymes may have potential biomedical applications as therapeutic agents for the detoxification of H2O2 and may give further information about the role of metal cofactors during the dismutation process. In this work, we present the synthesis and characterization of manganese(II) and oxomanganese(IV) complexes of a pentadentate ligand (N4Py* = N,N-bis(2-pyridylmethyl)-1,2-di(2-pyridyl)ethylamine), [MnII(N4Py*)(CH3CN)](CF3SO3)2 (1) and [MnIV(O)(N4Py*)](CF3SO3)2 (2). Detailed pH-potentiometric titrations were also performed in order to gain insights into the complexation properties of the N4Py* ligand, and the equilibrium model used for fitting the pH-potentiometric titration data for the [Mn(N4Py*)]2+ complex was confirmed by 1H-NMR relaxometry at 0.49 T field strength and 25 °C. Complex 1 has been shown to catalyze the dismutation of H2O2 into O2 and H2O in both aprotic (MeCN and DMF) and protic (TFE and buffered H2O) solvents. The reactivity of 1 was higher in protic solvents, which was markedly influenced by the pH, and it revealed a Michaelis–Menten behaviour at pH 10.5 at 298 K. A mononuclear oxomanganese(IV) complex, 2, as a possible intermediate in the catalytic process, was also prepared, and its stability and reactivity towards H2O2 was also investigated in TFE and buffered H2O/TFE solutions. The stoichiometric oxidation of H2O2 with 2 provided clear evidence (solvent isotope effect (SIE) of 6.2, pH dependence, saturation kinetics, etc.) of the rate-determining hydrogen atom transfer (HAT) mechanism between the H2O2 and oxomanganese(IV) species. The present results provide one of the first examples of a non-heme MnIVO complex that shows a catalase-like reaction in water.


 Please wait while we load your content...
Please wait while we load your content...