Strong intramolecular DyIII–DyIII magnetic couplings up to 15.00 cm−1 in phenoxyl-bridged dinuclear 4f complexes†
Abstract
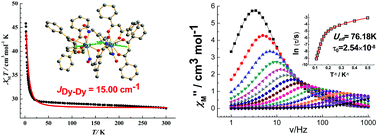
Three phenoxyl-bridged dinuclear lanthanide complexes [Ln2(dbm)2(LH2)2]·H2O (Ln = Gd for 1, Tb for 2, and Dy for 3, LH3 = (1E,3E)-2-hydroxy-5-methylisophthalaldehyde dioxime, Hdbm = dibenzoylmethane) were synthesized, and structurally and magnetically characterized. In these complexes, LnIII ions are eight coordinated and lie in D4d local symmetry. Intramolecular ferromagnetic couplings are exhibited between LnIII ions. Surprisingly, the intramolecular DyIII–DyIII magnetic couplings are up to 15.00 cm−1, which are the strongest ones of the reported Dy2 complexes by phenoxyl bridges. Complex 3 shows single-molecule magnet behavior with an effective energy of 76.18 K under zero dc field which is in accordance with the calculated value. The magnetostructural correlation of phenoxyl-bridged Dy2 complexes is analyzed.


 Please wait while we load your content...
Please wait while we load your content...