Shedding light on the bonding situation of triangular and square heterometallic clusters: computational insight†
Abstract
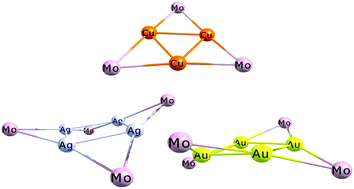
The motivation of this paper is to provide a comprehensive computational study of a series of heterometallic clusters (1a–3e) [MMoCp(CO)3]n (M = Cu+, Ag+ or Au+, n = 3 or 4); (Cp = η5-C5H5), seeking to clarify the effect of the coinage metal employed on the nuclearity observed, as well as to examine the role of metallophilicity in the cluster stability. A DFT benchmark revealed that the best results are obtained by using the D3ECP model, more specifically with the B3PW91-D3 functional. The calculated structures reproduce the experimentally observed distortion of the Ag4 core adequately, with bond lengths close to experimental values. Energy decomposition analysis, employing three different fragmentation schemes, revealed that the bonding situation is mainly modulated by electrostatic interactions, followed by orbital contributions, while the presence of dispersion is small, but not negligible. The EDA results for FSIII reveal that for the copper complexes the interaction energies are very similar for both the triangular and square cores, while for the complexes containing square metallic cores of silver or gold, they are much more stabilizing than for the triangular analogues. The density flow channels indicated that the orbital stabilization in the gold complexes is mainly from 4d(Mo) + π(CO) → 6s(Au) donations and 5d(Au) → π*(CO) + 4d(Mo) + σ(CH) back-donations. The inner-fragment polarization also contributes to the bonding stabilization.


 Please wait while we load your content...
Please wait while we load your content...