Synthesis, photophysics and biomolecule interactive studies of new hybrid benzo-2,1,3-thiadiazoles†
Abstract
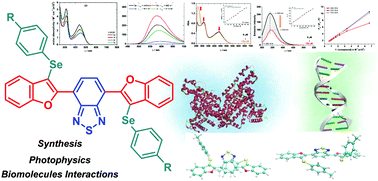
Four new hybrid molecules containing benzo-2,1,3-thiadiazole, benzofuran and arylselanyl moieties, named 4,7-bis(3-(arylselanyl)benzofuran-2-yl)benzo[c][1,2,5]thiadiazoles, were designed and synthesized in good yields through an electrophilic cyclization of 4,7-bis((2-methoxyphenyl)ethynyl)benzo[c][1,2,5]thiadiazole 1 and diaryl diselenides 2 using trichloroisocyanuric acid to generate electrophilic arylselanyl species. The photophysical properties of compounds 3a–d were investigated using UV-vis absorption and emission fluorescence analysis. The emission fluorescence analysis showed a yellow-orange region emission. Large Stokes shift values were observed for all new derivatives and attributed to the ICT state and to the electron-substituent properties. The biomolecule-binding DNA and HSA-binding interactions were also investigated by experimental and theoretical methods.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST


 Please wait while we load your content...
Please wait while we load your content...