Tuning the structure and photoinduced linkage isomerism of tetrapyridine nitrosyl ruthenium(ii) complexes by changing the trans-to-NO coordinated ligand†
Abstract
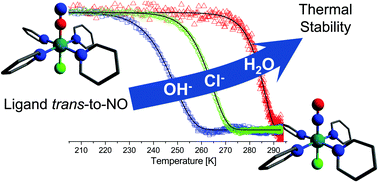
The perchlorate series of nitrosyl ruthenium tetrapyridine-cations trans-[RuNOPy4X]n+ (where X = OH, Cl, and H2O) were synthesized. The exchange of the anion in an excess of sodium perchlorate results in the formation of the hydroxo complex, with perchlorate as a counter-anion. The protonation of the coordinated hydroxyl resulted in the formation of the aqua complex in acidic media. The outcome of the protonation depends on the choice of the acid. The use of non-coordinating perchloric acid leads to the aqua complex as the product. The ruthenium nitrosyl complex with a coordinated chloride anion was a result of the interaction of the hydroxo complex with concentrated hydrochloric acid. The crystal structures were determined using X-ray single crystal analysis. The 445 nm irradiation promotes electron transfer from the metal orbital to the antibonding orbital of the nitrosyl group. In the solid phase, this metal-to-ligand charge transfer results in the formation of the metastable states with Ru–ON coordination. These photoinduced linkage isomers for each complex were monitored by IR-spectra and their stability was measured using the temperature sweep method described in an earlier study.


 Please wait while we load your content...
Please wait while we load your content...