Study on the internal electric field in the Cu2O/g-C3N4 p–n heterojunction structure for enhancing visible light photocatalytic activity†
Abstract
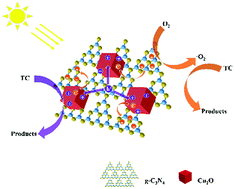
Study of the degradation mechanism of the Cu2O/g-C3N4 p–n heterojunction is very challenging and important for the preparation and practical applications of the g-C3N4 photocatalyst in controlling water pollution. Therefore, the Cu2O/g-C3N4 p–n heterojunction was designed and prepared successfully and the mechanism of enhanced visible-light photocatalytic activity was proposed. The morphology and structure of the synthesized materials were characterized by using SEM, TEM, FT-IR and XPS. And the optical and electrical properties of the materials were studied by measuring DRS, the photocurrent density and the Mott–Schottky curve. The results showed that the g-C3N4 photocatalyst is coated on the surface of Cu2O to form a tight heterojunction, which facilitates the separation and migration of photogenerated carriers, and the quantum yield of the Cu2O/g-C3N4 composite photocatalyst was 3–7 times higher than that of the single component. It has been shown that the built-in electric field formed at the heterojunction interface can effectively separate photogenerated electrons and holes. The photocatalytic performance of the material was evaluated by the photocatalytic degradation of tetracycline (TC) under visible light. The maximum degradation rate of Cu2O/g-C3N4 for TC could reach over 90% in 90 min, and the degradation rate of the single component is more than doubled. Simultaneously, the TOC tests monitored the mineralization of TC up to 83.4%, and the active substances in the reaction process were determined to be ˙O2− and h+ through free radical trap experiments and ESR tests. This work shows that the construction of an internal electric field has greatly improved the photocatalytic performance of g-C3N4. The Cu2O/g-C3N4 p–n heterojunction has good degradation ability toward tetracycline and is a good tetracycline-type wastewater treatment material.


 Please wait while we load your content...
Please wait while we load your content...