Regioselective synthesis of ortho-iodobiphenylboronic acid derivatives: a superior catalyst for carboxylic acid activation†
Abstract
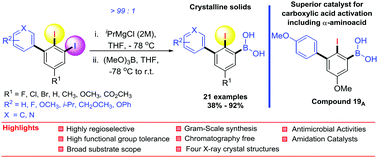
An efficient and versatile synthesis of ortho-iodobiphenylboronic acids via the highly regioselective metal–iodine exchange (MIE) of 2,3-diiodobiphenyls is reported. The site-selectivity is very much controlled by the size of the biphenyl fragment, providing only the terminal arylboronic acid derivatives in excellent site-selectivity. The nature of the substituents (R1 and R2) on the biphenyls is found to have an influence on the reactivity but not on the regioselectivity. The best reactivity and the highest isolated yields were furnished with products bearing electron-donating groups. The synthesized derivatives were also tested for in vitro antimicrobial activity against four strains of bacteria and one fungal strain. This revealed that (2-iodo-4′-isopropyl-[1,1′-biphenyl]-3-yl)boronic acid 6A and (3-(benzo[d][1,3]dioxol-5-yl)-2-iodo-5-methoxyphenyl)boronic acid 22A possess the most potent antibacterial and antifungal activity with MICs of 0.10 and 0.3 mg mL−1 for B. cereus and C. albicans respectively. The catalytic activity was also examined towards an amidation reaction at ambient temperature and this revealed a new optimal catalyst, (2-iodo-4′,5-dimethoxy-[1,1′-biphenyl]-3-yl)boronic acid 19A providing a remarkable increase in the amide yields, including α-aminoacids. This work discloses a protocol for the first synthesis of hitherto unknown ortho-iodobiphenylboronic acid derivatives that is scalable, and general in scope, where no chromatographic purification is necessary and the products are indeed potential organocatalysts.


 Please wait while we load your content...
Please wait while we load your content...