Catalytic systems based on nickel(ii) complexes with bis(3,5-dimethylpyrazol-1-yl)methane – impact of PPh3 on the formation of precatalysts and selective dimerization of ethylene†
Abstract
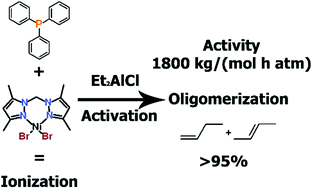
This study was aimed at elucidating the role of Ph3P in the formation of Ni(II) complexes which are active in ethylene oligomerization. Two ionic Ni(II) complexes bearing bis(3,5-dimethylpyrazol-1-yl)methane ligand [NiL2(CH3CN)2]2+[NiBr3(PPh3)]2− and [NiL2Br]+[NiBr3(PPh3)]− have been synthesized. The structures of these compounds have been confirmed by X-ray diffraction. Individual and in situ complexes with PPh3 are in equilibria between different forms in solution. These forms include the free complex and free PPh3, and molecular and ionic complexes with coordinated triphenylphosphine. All individual compounds were active in ethylene dimerization upon activation with Et2AlCl, producing a mixture of butenes with activities up to 960 kg mol[Ni]−1 h−1 atm−1 and high selectivity (up to 100% of butenes and up to 90.5% of butene-1). The catalytic influence of triphenylphosphine has been evaluated – it increases the activity of the system up to 1800 kg mol[Ni]−1 h−1 atm−1 when 2 mol. equiv. of additive has been applied and completely changes the selectivity of the process towards internal olefins.


 Please wait while we load your content...
Please wait while we load your content...