Synthesis, characterization and in vitro anticancer activity of thiabendazole-derived 1,2,3-triazole derivatives
Abstract
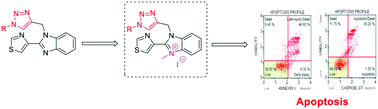
A series of thiabendazole-derived 1,2,3-triazole compounds were synthesized in good yields via 1,3-dipolar cycloaddition through click chemistry approach using different chain alkyl azides with 4-(1-(prop-2-yn-1-yl)benzimidazole-2-yl)thiazole in the presence of copper(I) catalyst. These compounds were characterized on the basis of FT-IR, 1H NMR, 13C NMR as well as mass spectrometry. The newly synthesized compounds were screened for their in vitro antiproliferative activity against a panel of three human cancer cell lines (HT29, MDA-MB-231 and SKBR3). Compound 4g exhibited significant activity against all the cell lines tested, with IC50 values ranging from 1.28 and 7.72 μg mL−1, inducing caspases 3 and 7, which further confirms the contribution of apoptotic cell death in MCF-7 and MDA-MB-231 cells. This work further demonstrates the anticancer properties of 4g, inducing apoptotic cell death, and the structure activity association is also discussed. This product could be a promising chemotherapeutic agent for cancer treatment.


 Please wait while we load your content...
Please wait while we load your content...