Synthesis of the first chiral polynuclear copper(i) complex based on (R)-1-(1-phenyl)ethyl-3-(O,O-diethylthiophosphoryl)thiourea and its characterization in the solid state and solution†
Abstract
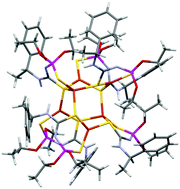
The reaction of (R)-1-(1-phenyl)ethyl-3-(O,O-diethylthiophosphoryl)thiourea with Cu(II) acetate is accompanied by copper reduction and leads to the formation of a chiral polynuclear Cu6L6 complex. In the crystalline phase the complex consists of six Cu(I) ions alternating with sulfur atoms in the central core, with all six ligands exhibiting similar 1,5-S,S-coordination modes. This structural multinuclear arrangement is preserved in solutions, while at the same time partial disproportionation is observed with the formation of complexes of lower dimensionality. Synchronous redox processes are observed for all six copper ions in the complex in the solid state. The mechanism of copper reduction with the ligand itself as a reducing agent is proposed.


 Please wait while we load your content...
Please wait while we load your content...