Solvent- and concentration-induced self-assembly of an amphiphilic perylene dye†
Abstract
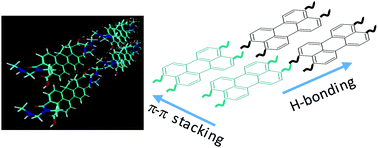
A new amphiphilic perylene dye bearing two carboxylic and two amidic chains (PDA-CA) has been synthesized and the ability to form π–π driven aggregates or folded structures has been investigated in aqueous or organic solvents at different concentrations, by means of NMR spectroscopy, theoretical calculations and optical characterization. The 1H NMR data showed the coexistence of supramolecular aggregates due to a synergetic effect of π–π stacking, hydrogen bonding and hydrophobic/hydrophilic interactions. The ratio between these species has been evaluated by concentration- and temperature-dependent 1H NMR experiments and also by the effect of aqueous or organic solvents. UV-Vis measurements are in agreement with NMR data evidencing the presence of more organized structures in organic solvents and aggregated species in aqueous solution. The π-stacking ability and the role intermolecular hydrogen bonds in the formation of different aggregated structures, was also estimated by density functional theory.


 Please wait while we load your content...
Please wait while we load your content...