Tunable electrochemiluminescence properties of CsPbBr3 perovskite nanocrystals using mixed-monovalent cations†
Abstract
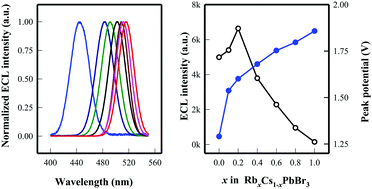
Herein, we demonstrate a simple approach for tuning the elecrochemiluminescence (ECL) properties of CsPbBr3 perovskite nanocrystals (NCs) by using mixed-monovalent cations. By replacing some of the Cs+ with Rb+, a series of RbxCs1−xPbBr3 NCs were obtained. They exhibit a narrow ECL emission peak with a full width at half-maximum of about 26–34 nm. After continuously injecting holes to produce different charged state radicals, an annihilation ECL was observed, implying that the as-prepared RbxCs1−xPbBr3 NCs are in an electron-rich state. In the presence of 2-dibutyaminoethanol as a co-reactant, strong anodic ECL of RbxCs1−xPbBr3 NCs was obtained. From CsPbBr3 NCs to RbPbBr3 NCs, the ECL peak potential position moved from 1.25 V to 1.80 V with the ECL spectrum peak shifting from 512 to 468 nm, respectively, indicating the tunable ECL properties. The Rb0.2Cs0.8PbBr3 NCs have a higher ECL intensity than the CsPbBr3 NCs.


 Please wait while we load your content...
Please wait while we load your content...