Self-oxygenated anatase–rutile phase junction: ensuring the availability of sufficient surface charges for photocatalysis†
Abstract
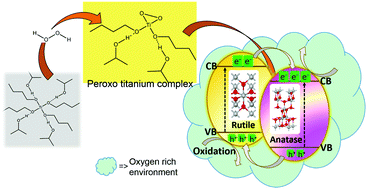
A peroxide assisted solvothermal strategy has been developed for the synthesis of self-oxygenated anatase–rutile mixed phase yellow TiO2. The yellow TiO2 possessed surface oxygen richness which has increased absorption to the visible region. The addition of peroxide to the isopropanol–titanium(IV) butoxide mixture paved the way for self-oxygenation. The self-oxygenated titania has enormous oxygen at its surface, and hence a negative charge cloud. This is beneficial for the selective degradation of cationic pollutants which is of great interest in contemporary society. This negatively charged surface of the self-oxygenated, anatase–rutile yellow titania mixed phase formed below the anatase to rutile transition temperature ca. 300 °C has facilitated more efficient solid–solid interfaces and thus interparticle charge transfer in photocatalysis. This strategy is directed towards more efficient photocatalysis of self-oxygenated mixed phase yellow TiO2 than that of the commercially available photocatalyst P25 titania.


 Please wait while we load your content...
Please wait while we load your content...