MnO2–GO-scroll–TiO2–ITQ2 as a low-temperature NH3-SCR catalyst with a wide SO2-tolerance temperature range†
Abstract
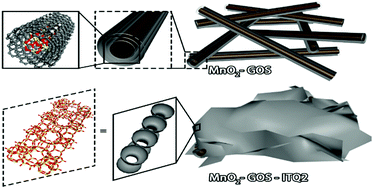
Three steps are needed to improve the steam-resistance and SO2-resistance of a catalyst for the selective catalytic reduction of NOx through NH3 at low temperature: the first is to introduce a protective layer to reduce the direct contact between SO3 and the catalyst. Then, there is delayed oxidation, which fundamentally reduces the oxidation of SO2 to SO3. If the catalyst is used at a relatively high temperature, it will inevitably produce SO3. The third step is to add a strong acid site in addition to reducing the acidity of the catalyst, which first absorbs NH3 and then absorbs SO3, to seize NH4HSO4, so that it does not cover the active site. GO was used to curl and wrap around the outside of MnO2 nanowires as a protective layer. TiO2 was selectively deposited on oxygen-containing functional groups on GO, which delayed the oxidation ability of the catalyst. ITQ2 molecular sieves acted as strong acid sites to absorb NH4HSO4. The curling behavior of GO outside MnO2 nanowires, the deposition location of TiO2 and the distribution of ITQ2 were explained by morphology and elemental analysis. In the range of 150 °C to 280 °C, the MnO2–GO-scroll–TiO2–ITQ2 catalyst conversion of NO to N2 was more than 85%. Combined with H2-TPR and activity testing, the source of the wide SO2-tolerance temperature range of the catalyst was described in detail.


 Please wait while we load your content...
Please wait while we load your content...