Phenothiazine and amide-ornamented novel nitrogen heterocyclic hybrids: synthesis, biological and molecular docking studies
Abstract
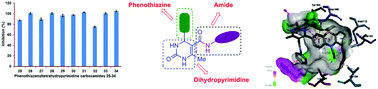
The synthesis of novel hybrids, namely, phenothiazine and amide-ornamented nitrogen heterocycles (25–34) has been accomplished utilizing a multi-step synthetic protocol and the structures have been established based on physical and spectral techniques. Of these, the hybrids possessing meta-nitro (26), para-fluoro (28), meta- and para-methyl (31), ortho-bromo (33) and ortho- and para-dimethyl (34) phenyl carboxamide scaffolds exhibited superior anti-inflammatory profiles over the standard diclofenac sodium. A hybrid integrated with a para-fluorophenyl carboxamide moiety (28) showed the highest DPPH radical scavenging activity among the chemical entities synthesized. Furthermore, the results of anticancer evaluations implied that the hybrid tethered with a phenyl carboxamide structural unit (29) exerted superior activity when compared with other hybrids against the pancreatic cancer cells SW1990 and AsPC1. Molecular docking between the hybrid 29 and B-cell lymphoma 2 reflects its appreciable binding affinity (−8.84 kcal mol−1). The results revealed that these chemical entities can serve as potent biological agents and/or efficient intermediates for the construction of potent biological agents.


 Please wait while we load your content...
Please wait while we load your content...