A one-pot route to N-acyl ureas: a formal four-component hydrolytic reaction involving aminonitrones and isocyanide dibromides†
Abstract
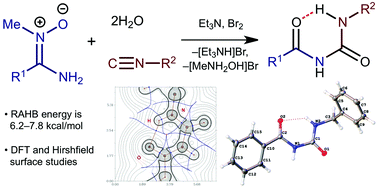
One-pot interplay between aminonitrones, isocyanides, dibromine, and water proceeds to give N-acyl ureas. This formally four-component reaction occurs via the initial generation of electrophilically activated 1,2,4-oxadiazolium salts, which then hydrolyze to grant N-acyl ureas. XRD structural and theoretical DFT studies indicated that the quasi-cyclic conformation of the N-acyl ureas is caused by moderate strength (6.2–7.8 kcal mol−1) intramolecular resonance-assisted hydrogen bonding, while their solid-state dimerization is determined by the collective action of intermolecular N–H⋯O (4.1–7.5 kcal mol−1) and C–H⋯O (1.6–4.7 kcal mol−1) hydrogen bonding. The results of the kinetic study accompanied by DFT calculations show that the generated 2-substituted 1,2,4-oxadiazolium salts are, as expected, significantly more reactive toward nucleophilic addition than the corresponding 1,2,4-oxadiazoles.


 Please wait while we load your content...
Please wait while we load your content...