Convenient synthesis of quinoline-fused triazolo-azepine/oxepine derivatives through Pd-catalyzed C–H functionalisation of triazoles†
Abstract
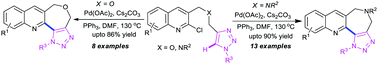
The efficient and convenient synthesis of a new fused heterocyclic scaffold comprising three different heterocycles, viz., quinolines, azepines/oxepines and triazoles is reported from quinoline-tethered triazoles. The quinoline-tethered triazoles were easily obtained in three steps from 2-chloro-3-formylquinoline, that is, reductive amination with benzyl amines and N-propargylation, followed by the ‘click’ reaction under standard conditions. For the first time, we present quinoline-fused triazolo-azepines in good to high yields by palladium-catalysed C–H functionalisation at the C-5 position of the triazole moiety. The protocol readily extended even to the construction of quinoline-fused triazolo-oxepines from (2-chloroquinolin-3-yl)methanol via a similar sequence, that is, O-propargylation, click reaction and palladium catalysed C–H functionalisation.


 Please wait while we load your content...
Please wait while we load your content...