A simple D–π–A system of phenanthroimidazole-π-fluorenone for highly efficient non-doped bipolar AIE luminogens: synthesis, and molecular optical, thermal and electrochemical properties†
Abstract
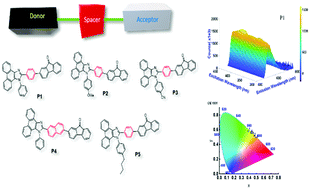
A series of hybrid organic bipolar fluorescent aggregation-induced emission (AIE) luminogens have been synthesized with D–π–A architecture using phenanthroimidazole as a conjugate by incorporating a fluorenone moiety at the C2 position of a rigid skeleton of 1,2-diphenyl-1H-phenanthro[9,10-d]imidazole. The synthesis was achieved by the Suzuki coupling reaction between 2-(4-bromophenyl)-1-phenyl-1H-phenanthro[9,10-d]imidazole and 2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-fluoren-9-one. All derivatives of the product were found to be thermally stable, with high melting and high decomposition temperatures. The phenanthroimidazoles P1–P5 displayed the natural properties of AIE. Wide colour contrast between the green and yellow regions was demonstrated by these luminogens. Among them, P1 displayed higher quantum yield and long lifetime properties. Thus, the obtained results clearly demonstrate that all the luminogens are promising materials for research and use in optoelectronic devices, sensing applications, biological probes and intercellular imaging.


 Please wait while we load your content...
Please wait while we load your content...