Revealing the potential application of chiral covalent organic frameworks in CO2 adsorption and separation†
Abstract
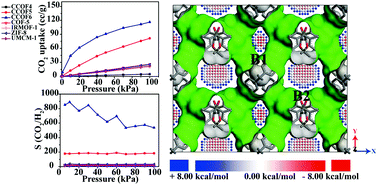
The adsorption and separation abilities of gases (including CO2, CH4, N2, and H2) in a series of chiral COFs (CCOFs) were studied using the Grand canonical Monte Carlo (GCMC) method, periodic density functional theory (DFT) (PBE functional) calculations and accurate dispersion corrected double-hybrid DHDF-D3 to explore the potential application of recently synthesized chiral COFs (Han et al., J. Am. Chem. Soc., 2018, 140, 892–895 and Han et al., J. Am. Chem. Soc., 2017, 139, 8693–8697). In contrast to four classical structures (including IRMOF-1, UMCM-1, COF-5, and ZIF-8), CCOF6 shows a better CO2 adsorption capacity at 298 K in a wide range of pressure from 0 to 100 kPa. More importantly, the selectivity of CO2 over N2, CH4 and H2 in CCOF6 is obviously greater than that of other classic porous materials. GCMC simulation demonstrates that the CO2 molecules prefer the smaller B1 channel in CCOF6 at pressure lower than 10 kPa; however, there is no obvious preferential adsorption site in CCOF5. To better understand the mechanism of CO2 adsorption in CCOFs, the interaction energy between CO2 and CCOFs was calculated by the double-hybrid DFT-D3, confirming the preferred adsorption sites in CCOF6 due to the proper narrow channel and the charge transfer from CO2 to CCOF6. Our well established theoretical calculation reveals that CCOF6, with promising CO2 adsorption and selectivity properties, has the potential to be an excellent material for CO2 adsorption and separation.


 Please wait while we load your content...
Please wait while we load your content...