Structures of the solvated copper(ii) ion in ammonia at various temperatures†
Abstract
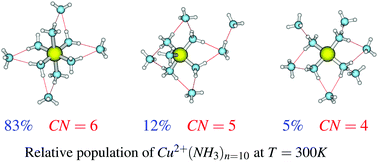
We investigated theoretically the structures and relative stabilities of the solvated copper(II) ion in ammonia, Cu2+(NH3)n, n = 1–10. Besides, we reported the temperature dependence of the isomer distribution of the studied clusters. Distances dCu2+–N for the solvated copper(II) ion in ammonia were discussed. Several significant new isomers and new global minimum energy structures of these clusters were pointed out. A satisfactory relative stability rule of the various isomers of this cluster is proposed and we expect it to be extended to other metal ions solvated in other media. It is worth mentioning that the only possible coordination numbers of the solvated copper(II) ion in ammonia are 4, 5 and 6 and these coordination numbers are strongly influenced by the temperature. The theoretical approach used in this work leads to equatorial distances dCu2+–N, in excellent agreement with experimental results. As far as the temperature dependence of the isomer distribution is concerned, compact and highly coordinated structures dominate the population of the studied clusters at all temperatures. For n = 2–5, the most stable isomers dominate exclusively the population of the clusters, irrespective of the temperature. For n ≥ 6, two to three isomers contribute to the population of the clusters. After reporting binding energies, clustering energies, clustering enthalpies and clustering free energies of the studied clusters, we proposed anticipated results for n > 10 through highly correlated fit functions with very low root mean square errors. We also confirm here our previous claim that clustering free energies at modest or higher cluster sizes (n > 7) for solvated metals could not depend on the metal type (alkaline earth, transition metals, …).


 Please wait while we load your content...
Please wait while we load your content...