Efficient solvent- and temperature-tuned access to aldoxime ethers and phenolic functions by Pd-catalyzed C–O cross-coupling of aldoximes with aryl bromides and bromo-chalcones†
Abstract
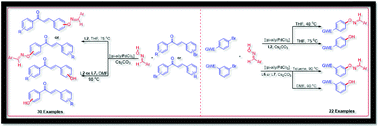
A single method with a functionality switching option was developed for the first time for the Pd-catalyzed C–O cross-coupling of aryl bromides and bromo-chalcones with aldoximes. The ligand tBuXPhos (L2) was found to be an effective supporting ligand for the Pd-catalyzed coupling of aldoximes with bromo coupling partners. The functionality switching from oxime ethers to a phenolic or hydroxy group was driven by solvent or temperature. This method offers the products in good to excellent yields in short reaction times.


 Please wait while we load your content...
Please wait while we load your content...