Lutetium and yttrium complexes supported by an anilido-oxazoline ligand for polymerization of 1,3-conjugated dienes and ε-caprolactone†
Abstract
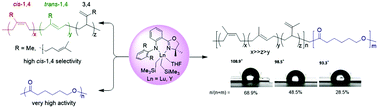
Lutetium and yttrium dialkyl complexes supported by an anilido-oxazoline ligand were synthesized, and their catalytic behaviors toward polymerization of isoprene (IP), β-myrcene (Myr) and ε-caprolactone (CL) have been explored. The treatments of [Ln(CH2SiMe3)3(THF)2] (Ln = Lu, and Y) with the anilido-oxazoline pro-ligands 2-(2,6-R2PhN)-phenyl-4-Me2-oxazoline (HLa: R = Me, and HLb: R = iPr) afforded the lutetium and yttrium dialkyl complexes [(La,b)Ln(CH2SiMe3)2THF] (La: Ln = Lu (1a), Y (2a); Lb: Ln = Lu (1b), Y (2b)). All the complexes not only exhibited high cis-1,4-selectivity for IP polymerization (87.0–95.9%) and Myr polymerization (82.3–94.5%), but were also found to be active in initiating ring-opening polymerization (ROP) of CL with outstanding activity (i.e., more than 97% yield within 1 min). Gratifyingly, when CL was added to a 100% conversion IP polymerization system catalyzed by 2b, diblock copolymer polyisoprene-b-polycaprolactone (PIP-b-PCL) could be isolated. By increasing the content of PCL in the copolymers, the static contact angle of water of the copolymers changed from 108.9° to 93.3°.


 Please wait while we load your content...
Please wait while we load your content...