Isoselective ring-opening polymerization and asymmetric kinetic resolution polymerization of rac-lactide catalyzed by bifunctional iminophosphorane–thiourea/urea catalysts†
Abstract
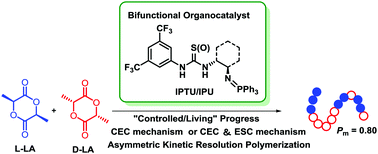
A series of iminophosphorane–thiourea/urea bifunctional catalysts was synthesized and utilized for the isoselective ring-opening polymerization of rac-lactide. The ROPs were promoted efficiently, affording the polylactides with controlled molecular weight, narrow molecular weight distribution and well-defined end groups without undesired side reactions. The experiment data revealed that ROPs of rac-LA are a “controlled/living” process. The highest stereoselectivity (Pm) was up to 0.80 using rac-IPU-1 under mild reaction conditions. The mechanistic study indicated that stereoselective ROPs of rac-LA were mainly controlled by a chain-end control mechanism when using rac/(R,R)-IPTU-1/IPU-1 as catalysts. Additionally, the ee values of −11% at 0 °C and −17% at −40 °C were achieved at about 50% conversion using (S)-IPU-2 as a chiral catalyst. The selectivity factor (s = kL/kD) of 1.6 indicated that a clear kinetic resolution occurred and the enantiomorphic site control mechanism was involved.


 Please wait while we load your content...
Please wait while we load your content...