The direct C–H alkenylation of quinoline N-oxides as a suitable strategy for the synthesis of promising antiparasitic drugs
Abstract
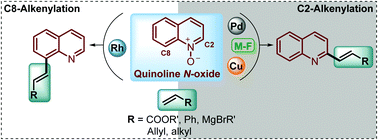
Functionalized quinolines are an important group of heterocyclic molecules with diverse, different, and interesting biological and pharmacological activities. Traditionally, several methods have been developed for the construction of this N-heterocyclic ring, but nowadays the functionalization of the quinoline scaffold has gained great interest and it is highly demand for the synthesis of quinoline derivatives. In this context, quinoline N-oxides have attracted considerable attention as a starting material for different transformations in organic chemistry, such as oxidations, deoxygenations, nucleophilic reactions, cycloadditions, aminations, etc. However, with the current need to extend the quinoline framework through the formation of new C–C bonds, in this review we will survey the recent developments in the direct alkenylation of quinoline N-oxides via the C(sp2)–H bond activation process, discussing metal-free and transition-metal catalysed protocols, and the regioselectivity during the synthesis of promising antiparasitic drugs.
- This article is part of the themed collection: 2019 Focus and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...