Chemical repair mechanisms of damaged tyrosyl and tryptophanyl residues in proteins by the superoxide radical anion†
Abstract
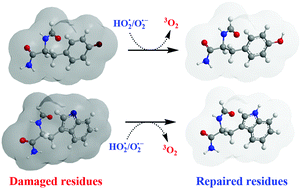
This article reports a computational kinetic and thermodynamic study of the chemical repair of radical-damaged tryptophan and tyrosine residues by the superoxide radical anion (O2˙−)–hydroperoxyl radical (HO2˙) pair, via single electron transfer (SET) and formal hydrogen transfer (FHAT) mechanisms. It was demonstrated that O2˙− can repair oxidized tyrosyl and tryptophanyl damaged residues, mainly by electron transfer, restoring them to their pristine structure. Acid–base equilibria were considered, and the influence of the pH on the main reaction mechanism was explored. The results presented here are expected to contribute to the better understanding of the complex and dual behaviour of the HO2˙–O2˙− in the context of oxidative stress.
- This article is part of the themed collection: Celebrating recent chemical science in Mexico


 Please wait while we load your content...
Please wait while we load your content...