First principle studies on the atmospheric oxidation of HFC-C1436 initiated by the OH radical†
Abstract
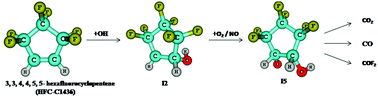
The atmospheric oxidation mechanism and kinetics of 3,3,4,4,5,5-hexafluorocyclopentene (HFC-C1436) initiated by the OH radical have been investigated using electronic structure calculations. Canonical variation transition state theory was employed to predict the rate constant for the favourable OH-addition reaction pathway. Under atmospheric conditions, the initially formed HFC-C1436-OH adduct, reacts rapidly with O2 and NO˙ to form the peroxy and alkoxy radical, which subsequently reacts with HO2 and O2 to form 2,2,3,3,4,4-hexafluoro-5-hydroperoxy-cyclopentanol (P1), 3,3,4,4,5,5-hexafluoro-cyclopentane-1,2-diol (P2) and 2,2,3,3,4,4-hexafluoro-5-hydroxy-cyclopentane (P3). Two different ring opening reaction pathways for the alkoxy radical were studied. In addition to the products, P1, P2 and P3, the possible end products identified from the ring opening reactions are CO, CO2, COF2 and glyoxal which will induce reduced environmental impacts compared to the chlorofluorocarbons (CFCs). The rate constant calculated for the favourable initial reaction at 298 K is 0.842 × 10−13 cm3 molecule−1 s−1 and is in agreement with the available experimental value. The atmospheric lifetime, global warming potentials (GWPs) for different time horizons and the photochemical ozone creation potential (POCP) were also calculated.


 Please wait while we load your content...
Please wait while we load your content...