A quasi-solid composite electrolyte with dual salts for dendrite-free lithium metal batteries†
Abstract
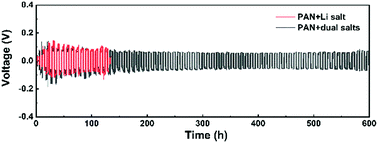
Lithium-ion batteries (LIBs) have been widely used in portable electronic devices and electrical vehicles. However, the LIBs with flammable organic liquid electrolytes have safety issues. Solid electrolytes have many advantages compared with organic liquid electrolytes, especially in terms of their low flammability and wide operation temperatures. In particular, the composite polymer electrolytes combining the advantages of the polymer and the inorganic electrolytes have good interfacial contact and high ionic conductivity. In this work, the composite electrolyte membranes consisting of a polyacrylonitrile (PAN)-Li6.5La3Zr1.5Ta0.5O12 (LLZTO) matrix as well as LiClO4 and Mg(ClO4)2 dual salts have been prepared by electrospinning. It is found that the cycle stability of the battery can be greatly improved by adding magnesium salt to the electrolyte membrane. The magnesium salt promotes the decomposition of LiPF6 in the electrolyte to produce fluoride ions. Thus, a stable protective layer of magnesium fluoride is formed on the surface of the lithium anode, which can effectively inhibit the growth of lithium dendrites, reduce the interface impedance, and increase the cycle life of the battery. Furthermore, the prepared lithium metal battery is also used to store mechanical energy harvested by triboelectric nanogenerators (TENGs).


 Please wait while we load your content...
Please wait while we load your content...