Enantioseparation using carboxymethyl-6-(4-methoxybenzylamino)-β-cyclodextrin as a chiral selector by capillary electrophoresis and molecular modeling study of the recognition mechanism†
Abstract
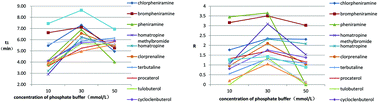
In this study, carboxymethyl-6-(4-methoxybenzylamino)-β-cyclodextrin (CMCDPN) was synthesized for the first time and managed to be used as a chiral selector to enantioseparate 13 kinds of chiral drugs (chlorpheniramine, brompheniramine, pheniramine, homatropine, homatropine methyl, clorprenaline, terbutaline, procaterol, tulobuterol, cycloclenbuterol, propranolol, pindolol and ofloxacin) by capillary electrophoresis (CE). The analysis was carried out by CE using a fused-silica capillary (50 cm × 50 μm i.d., 40 cm effective length) with 30 mmol L−1 phosphate buffer under an applied voltage of 20 kV. The effect of analysis conditions such as buffer concentration, buffer pH, applied voltage and concentration of the chiral selector on the enantioseparation results was systematically investigated. By the method established by us, eleven of the analytes reached baseline separation and the other two reached partial separation. The molecular dockings were applied to further demonstrate the mechanisms of chiral recognition. The docking results showed good agreement with our experimental results.


 Please wait while we load your content...
Please wait while we load your content...