Unraveling the role of the lacunar Na7PW11O39 catalyst in the oxidation of terpene alcohols with hydrogen peroxide at room temperature†
Abstract
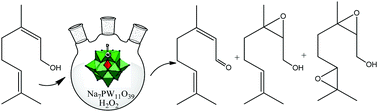
In this work, we have assessed the activity of various Keggin heteropolyacid (HPA) salts in a new one-pot synthesis route of valuable products, which were obtained from the oxidation of terpenic alcohols (i.e., aldehyde, epoxide, and diepoxide), using a green oxidant (i.e., hydrogen peroxide) at mild conditions (i.e., room temperature). Lacunar Keggin HPA sodium salts were the goal catalysts investigated in this reaction. Starting from the HPAs (H3PW12O40, H3PMo12O40, and H4SiW12O40), we synthesized lacunar sodium salts (Na7PW11O39, Na7PW11O39 and Na8SiW11O39) and a saturated salt (Na3PW12O40). All of them were investigated in oxidation reactions in a homogeneous phase with nerol as a model molecule. Na7PW11O39 was the most active and selective towards the oxidation products. All the catalysts were characterized by FT-IR, TG/DSC, BET, XRD, and SEM-EDS analyses and potentiometric titration. The main reaction parameters were assessed. Geraniol, α-terpineol, β-citronellol and borneol were also successfully oxidized. Special attention was dedicated to correlating the composition and properties of the catalysts with their activity.


 Please wait while we load your content...
Please wait while we load your content...