How does the acidic milieu interfere in the capability of ruthenium nitrosyl complexes to release nitric oxide?†
Abstract
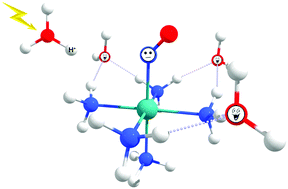
The nitric oxide (NO) molecule is involved in a large number of biological routes. Thus, there is increasing interest in improving the understanding of the NO release mechanisms. One of the traditional NO release mechanisms involves (i) [Ru(NO)(NH3)5]3+ + e− → [Ru(NO)(NH3)5]2+ and (ii) [Ru(NO)(NH3)5]2+ + H2O → [Ru(H2O)(NH3)5]2+ + NO chemical reactions. Another possibility is associated with light irradiation: (iii) [Ru(NO)(NH3)5]3+ + H2O + hν → [Ru(H2O)(NH3)5]3+ + NO, aided by the Ru(dπ) → π*(NO) electronic transition, which decreases the π back-donation process in the Ru–NO chemical bond. The influence of the acid environment in which these chemical reactions typically occur experimentally has been explored in (iv) [Ru(NO)(NH3)5]2+ + H3O+ → [Ru(HNO)(NH3)5]3+ + H2O; and (v) [Ru(HNO)(NH3)5]3+ + H2O → [Ru(H2O)(NH3)5]3+ + HNO reactions. Reaction (v), supported by eight explicit water molecules, is the most propitious to occur. The HNO charge obtained from the atomic polar tensor scheme is close to zero. The methods of quantum theory of atoms in molecules and non-covalent interactions reveal that the HNO leaving group interacts with two water molecules through partially covalent or ionic chemical bonds. The HNO → NO conversion after the release from ruthenium molecules is thermodynamically feasible. The electronic spectrum of the structure [Ru(HNO)(NH3)5]3+ has, unlike the [Ru(NO)(NH3)5]3+ molecule, the Ru(dπ) → π*(NO) transition with an appropriate absorbance. Therefore, the proton increases the capability of ruthenium complexes to release nitric oxide after the chemical reduction reaction or the light-supported chemical reaction.


 Please wait while we load your content...
Please wait while we load your content...