Synthesis of a monofunctional glycoluril molecular clip via cyclic imide formation on the convex site†
Abstract
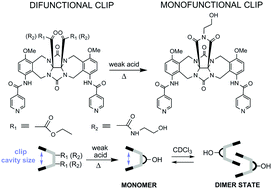
Selective homodimerization of glycoluril molecular clips makes this class of molecules valuable supramolecular motifs with great potential in the area of reversibly cross-linked self-healable polymer materials. However, their introduction into a polymer matrix requires suitable functionalization on the clip convex site, which does not participate in the association processes. Generally, the glycoluril clip's convex site is equipped with two ester groups, 1. The presence of two functional groups is usually undesired during the polymerization process or any functionalization reactions. A monofunctionalized glycoluril clip would assure targeted and convenient clip introduction into a polymer structure. Here, we present the successful synthesis of a glycoluril clip with a monohydroxylated convex face, 3, which was easily obtained from the dihydroxyl clip derivative, 2, under mild conditions, in the cyclization reaction of diethanolamide groups into an N-(2-hydroxyethyl) five-membered imide, applying a catalytic amount of weak organic acids. Experimental data supported by computer simulation facilitated determination of optimal conditions of a new clip formation. Moreover, we demonstrated a possibility of a direct conversion of a diester clip, 1, into a clip, 3, eliminating one reaction step. The structure of the glycoluril clip 3 was confirmed using ESI-MS, and 1D and 2D NMR spectroscopy. DFT calculations carried out for clips equipped with diester, diethanolamide and monohydroxylated cyclic imide groups, respectively, revealed that upon the clip's convex functionalization, including cyclization, the size of the clip's cleft does not change, which is of great importance in view of the clip association ability. Variable-temperature 1H NMR spectroscopy in the range from 223 to 323 K confirmed the ability of the new clip to dimerize, demonstrating its thermosensitive character. In addition, the clip exhibits high thermal stability up to 453 K, which is crucial in view of the synthesis and processing of clip-based polymer materials.


 Please wait while we load your content...
Please wait while we load your content...